|
|
ABCD nationwide IDegLira audit
About the ABCD nationwide
IDegLira
audit
This audit follows on from the success of the
previous ABCD nationwide audits of GLP1 receptor agonists, SGLT2
inhibitors, and
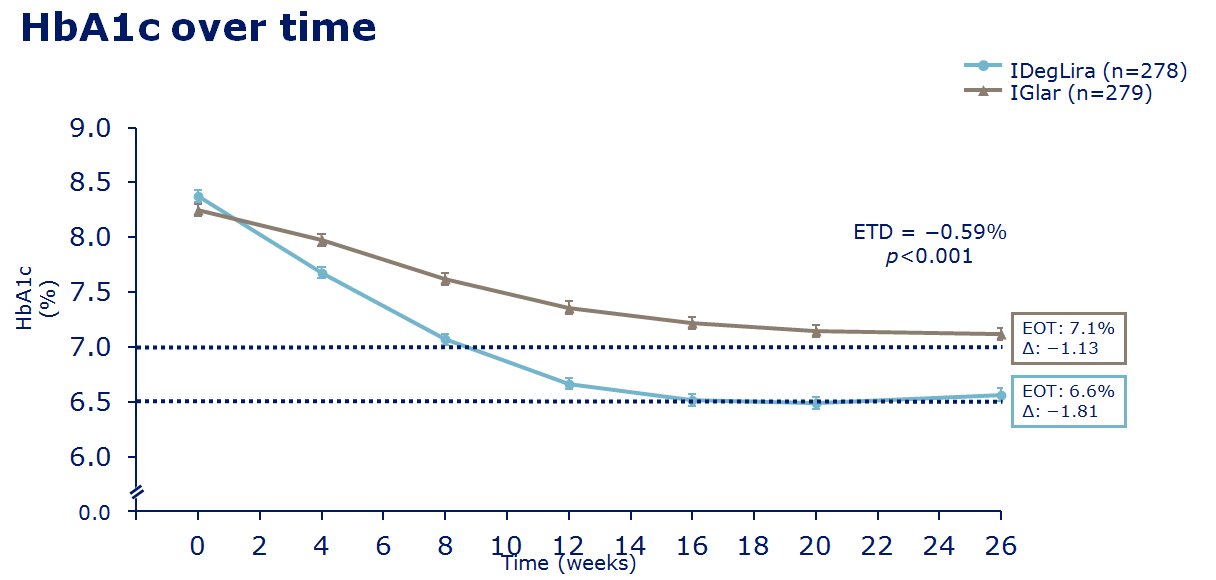
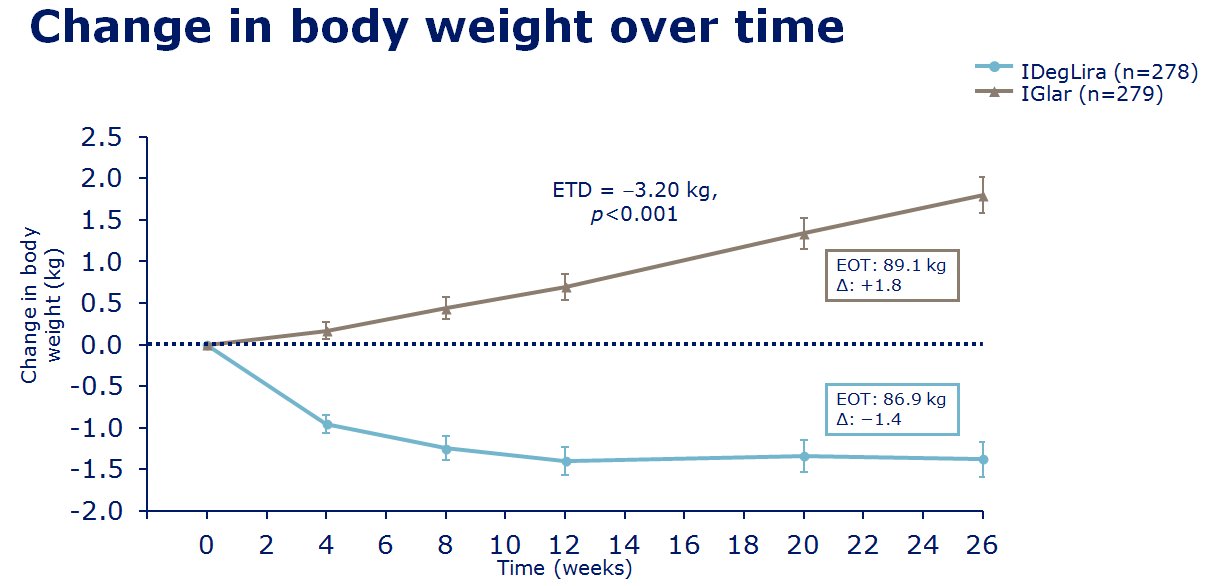
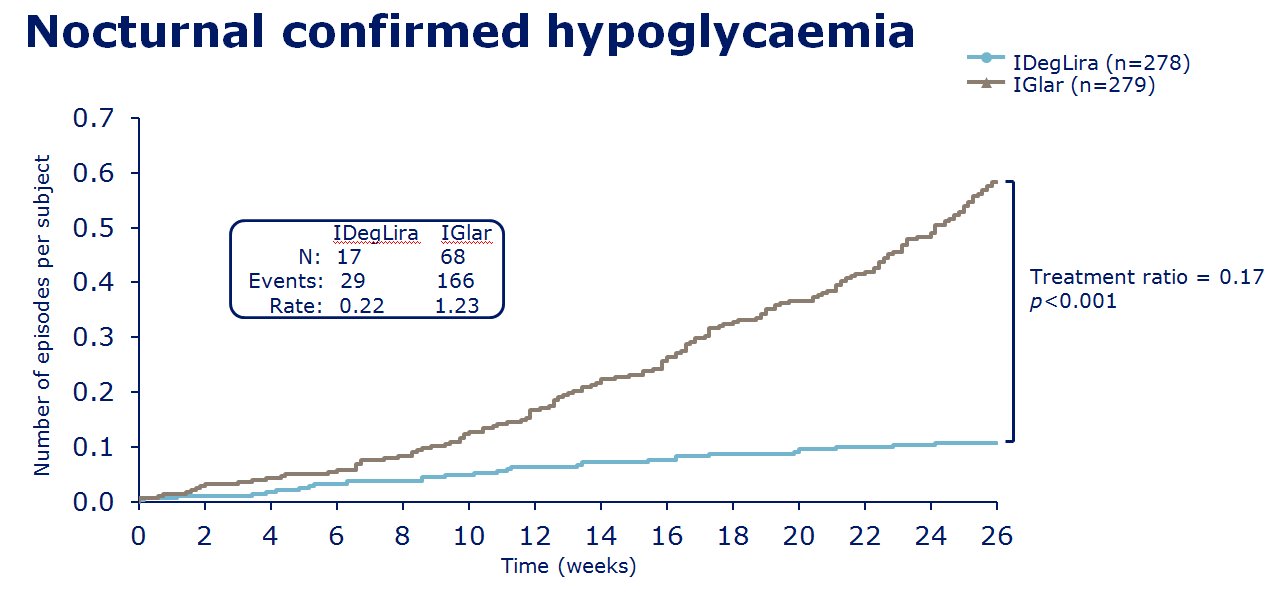
insulin degludec. The clinical trials of IDegLira seem to show in
those uncontrolled on basal insulin (20-50units), IDegLira showed
statistically improved HbA1c reductions in comparison to the up
titration of insulin glargine U100 with fewer hypoglycamic episodes and
less weight gain, and indeed with weight loss - see
slides on the left for examples of the data concerned. Also in clinical
trials, when iDegLira was compared to liragutide in patients
uncontrolled on OADs or to unchanged maximum tolerated GLP-1 (liraglutide
or exanetide bd) results showed statistically improved HbA1c and FPG
control with fewer gastrointestinal side effects but higher rates of
hypoglycaemia and less weight reduction in one trial and weight increase
in another. We hope through
this nationwide audit to find out if these findings from the clinical
trials translate into the same advantages when the agent is used in real
clinical practice. The audit will be hosted on a tool very similar to
that used in the
liraglutide audit and the
degludec audit so the many contributors taking part in those audits
will find it particularly easy. The audit will launch in February or
March, 2017, and has a number of
objectives.
Collect
data on-line or via paper forms
The IDegLira on-line audit tool is so
easy to use that live data entry in clinic is a real option
to be considered. Otherwise to facilitate data collection
during clinics there are two paper forms which exactly match
the data that can be entered into the audit tool. You can
download and print these forms locally or
order pre-printed data entry forms.
To download the forms to printout for use, use the
following links:
Download first visit data entry form
Download follow up visit data entry form
Non ABCD
members
Non ABCD members are welcome to take
part in the audit and will be given access to the on-line
audit tool when they
register
for the audit.
Register to take part in the audit and access to
the on-line tool
To register for the audit and be
given access to the on-line tool
click here.
Further
information
Further enquiries may be made to the ABCD nationwide audits
database administrator of the project,
Jayne Starrett |
Register for the audit
Access
the on-line tool
IDeglira audit objectives
Order
preprinted data entry forms
Download
first visit data entry form
Download
follow up visit data entry form
How
to analyse your data - video
Further
information- contact us
Main ABCD homepage
|