|
Many patients globally have combined
obesity and diabetes, or ‘diabesity’, including in the UK.
New, effective therapies are urgently needed. A newer group
of injectable non-insulin drugs, the ‘GLP-1 receptor
agonists’ can improve diabetes control with weight loss but
many patients fail to achieve treatment targets; bariatric
weight loss surgery is not a universal solution as it is not
without complications and not widely available. Furthermore,
the benefits of intense caloric restriction are difficult to
sustain. Hence the need for new treatments to
combat this problem of diabesity, which is rising at an
alarming rate. The endobarrier is one such treatment which
seems to be
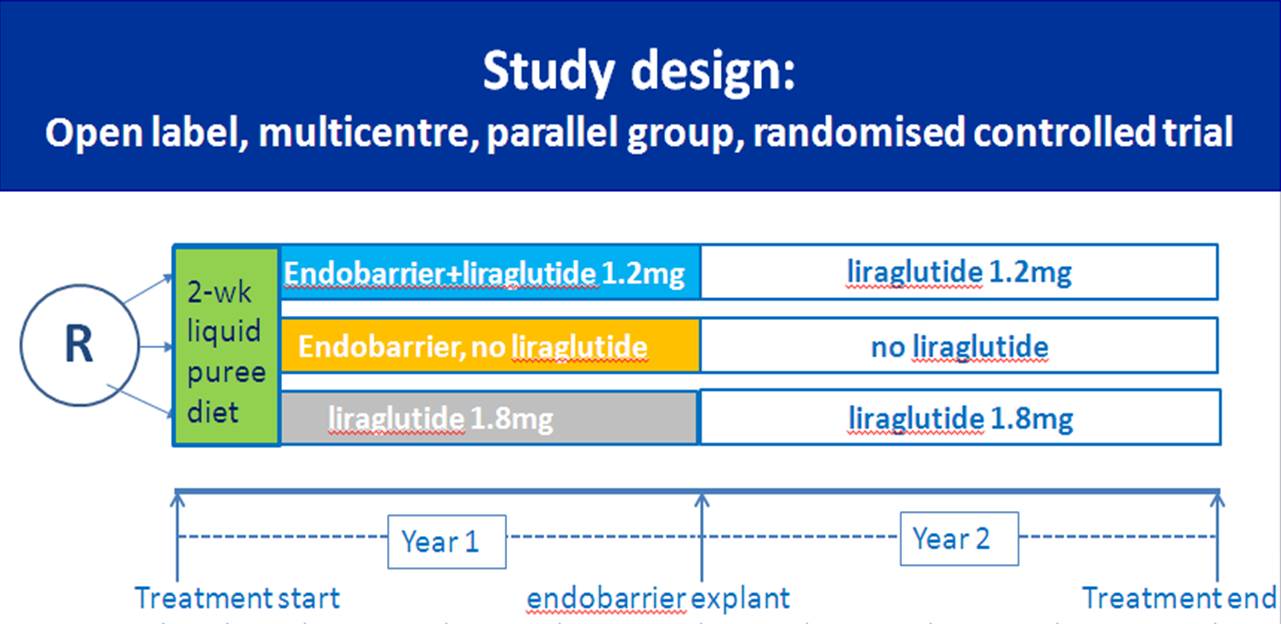
effective in clinical trials. The REVISE-Diabesity
trial aims to test its effectiveness in an NHS setting.
|