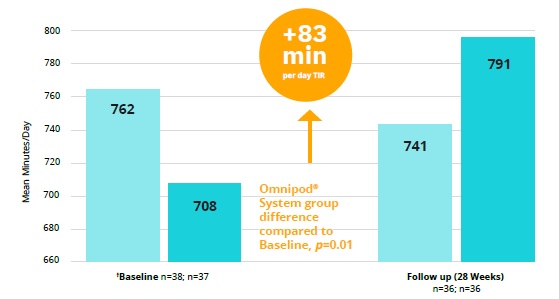
Above – In a randomised controlled trial, the Omnipod System improved
glycaemic control measured by time in the glucose range of 3·9–10·0 mmol/L
by an average daily proportion of 6% more than multiple daily insulin
injections (MDI) without any occurrences of severe hypoglycaemia.
However, biochemical hypoglycaemia also was increased. It will be of
interest to see what happens to these measurements when patients are
initiated on Omnipod in the real world. The audit should provide this
information
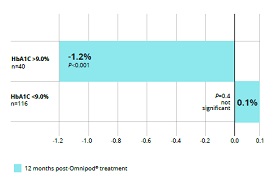
Above – In a study comparing adults switched from MDI to
Omnipod system to a matched cohort who maintained MDI, the Omnipod group
had a lower HbA1c and total daily insulin dose with significant
improvements in those with HbA1c≥75mmol/mol (≥9%). Will these findings
be reproduced when patients undergo the same switch in our real world
audit. The audit should provide this information
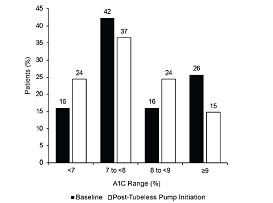
Above – A multicenter, retrospective study suggested
that initiating tubeless insulin pump therapy following transition from
either MDI or CSII with a tubed insulin pump was a associated with
significant improvement in HbA1C most notably in those with A1C ≥9.0%
and those previously treated with MDI. In this context it will be of
interest to see if these results will be replicated in the UK and
elsewhere in the world – is the same being found? The audit should
provide this information
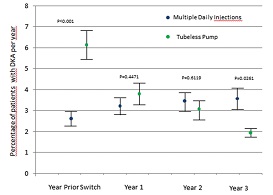
Above - German registry data suggested that that
tubeless insulin pump therapy is associated with good glycaemic control
and a low frequency of DKA and severe hypoglycaemia in an age group
prone to acute complications. What about in the UK and elsewhere in the
world – is the same being found? The audit should tell us
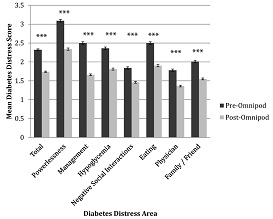
Above - A study in California of quality of life (QOL)
amongst Omnipod users found substantial QOL benefits among users. What
about QOL in real world users in the UK and elsewhere in the world –
will the same be found? The audit should tell us |
ABCD worldwide Omnipod audit
ABCD worldwide Omnipod audit
Following the success of the first audit of a device, the FreeStyle
Libre audit, this audit is the fourth device related audit and it is due
to launch before the end 2021. Insights into the sorts of helpful
information learned from previous ABCD audits that has helped improve
patient care in the past, can be gained through this link:
https://abcd.care/all-abcd-audit-publications, though this is by no
means comprehensive: Centres worldwide are invited to join the Omnipod
audit.
About the ABCD worldwide Omnipod audit
Omnipod is a tubeless insulin pump system. On the left you will find
examples of findings from published research suggesting improvements in
glycaemic control, acute diabetes complications and quality of life
associated with this technology rather than multiple daily insulin
injections or CSII with a tubed insulin pump. As with previous ABCD
audits both of therapies and devices, the aim of this audit is to
establish the extent to which such published findings will also be found
when the Omnipod system is used in real clinical practice in the UK and
elsewhere. The audit has a number of objectives.
Web-based audit tool
The audit tool for the Omnipod audit is similar to that being used for
the other ABCD audits. The tool is easy to use. All data is encrypted
and secured to a high level and is fully GDPR compliant meaning it has
optimum security for patient identifiable data with regard to your own
patients but anonymises the data when it is utilised in the national and
global audit. There are some special features with regard to the data
export both for your own local analysis and for the nationwide analysis.
The export now allows you to choose which data to download for analysis
as well as providing all data. It also allows you to choose to download
the data aggregated to different time points.
Structure of the audit – centres and sites
For this audit the concept of centres and sites is utilised in
the same way as in the other ABCD audits. Typically, a centre might be
an NHS Trust. Sites might be hospitals associated with that Trust,
and/or health centres or GP surgeries in the local vicinity. If set up
in this structure, designated leaders of the local audit would be given
access to download the anonymised data of all the patients associated
with the centre for more powerful local analysis of data involving
higher numbers. Findings so made through such local analysis could be
put forward for further testing on the full national dataset. Outside
the UK, instead of centres and sites, contributors are registered as
country and centre within that country.
Collect data on-line or via paper forms
The on-line audit tool will be so easy to use that live data entry in
clinic is a real option to be considered. Otherwise, to facilitate data
collection during clinics there are two paper forms which exactly match
the data that can be entered into the audit tool.
To download the forms to printout for use, use the following links:
Download first visit data entry form
Download follow up visit data entry form
Caldicott Guardian approval
The ABCD audit programme has Caldicott Guardian approval. The
programme is audit not research. The NHS encourages audit of clinical
practice and there are strict guidelines which we follow, in particular
that we only to collect data from routine clinical practice, and
analysis is of data which is anonymised.
Further information
Further enquiries may be made to the ABCD nationwide audits
database administrator of the project,
Melissa Cull.
|
Register for the Omnipod Audit
Access the on-line tool
Omnipod audit
objectives
Download first visit data entry form
Download follow up visit data entry form
Download first visit data entry form (editable)
Download follow up visit data entry form (editable)
Brief
Guide for international centres
Papers, abstracts, presentations, posters from the audit
Further information- contact us
ABCD on HSCN homepage
Main ABCD homepage
|