|
Current, live, ABCD nationwide
audits of new therapies and devices in real clinical practice
ABCD has learned from its previous nationwide audits. It
has learned the best ways of undertaking successful nationwide audits.
It has also realised how much clinically useful information can be
obtained from the audit information to improve patient care. This
applied in particular to the ABCD nationwide GLP1-RA audits of
exenatide
and
liraglutide. Similarly now we are gaining considerable
insight into the SGLT2 class. Many of our national audits are now on
N3 which is the latest version of NHSnet. The audits currently
being undertaken are:
Nationwide audits of new diabetes therapies and devices on N3
Nationwide FreeStyle Libre audit
The nationwide audit of the the FreeStyle Libre (FSL) glucose monitoring
system represents our first audit of a device. All using FSL
in adults and paediatric patients with either type 1 or type
2 diabetes are invited to join the audit.
The tool for the audit is currently being developed and should be
available soon.
In the meantime paper forms are available (click here to be sent the
forms) fso that you can start collecting data without delay in readiness
for when the on-line tool becomes available.
Click here for more information and
to join the audit.
Nationwide empagliflozin audit
The nationwide audit of the SGLT2 inhibitor, empaglflozin (Jardiance®) launched in
March 2017. All users of empagliflozin are invited to join the audit.
The tool provided for on-line data entry has a facility
for easy, sophisticated, analysis of the data entered by the local
centre entering the data. At the same time the data entered
automatically becomes available, in anonymised form, for the national
audit.
Click here for more information and
to join the audit.
Nationwide canagliflozin audit
The nationwide audit of the SGLT2 inhibitor, canaglflozin (Invocana®) launched in
January 2016. All users of canaglifozin are invited to join the audit.
The tool provided for on-line data entry has a facility
for easy, sophisticated, analysis of the data entered by the local
centre entering the data. At the same time the data entered
automatically becomes available, in anonymised form, for the national
audit.
Click here for more information and
to join the audit.
Nationwide dapagliflozin audit
The nationwide audit of the firs SGLT2 inhibitor available in the UK, dapaglflozin (Forxiga®) launched in
October 2014. All users of dapagliflozin are invited to join the audit.
The tool provided for on-line data entry has a facility
for easy, sophisticated, analysis of the data entered by the local
centre entering the data. At the same time the data entered
automatically becomes available, in anonymised form, for the national
audit.
Click here for more information and
to join the audit.
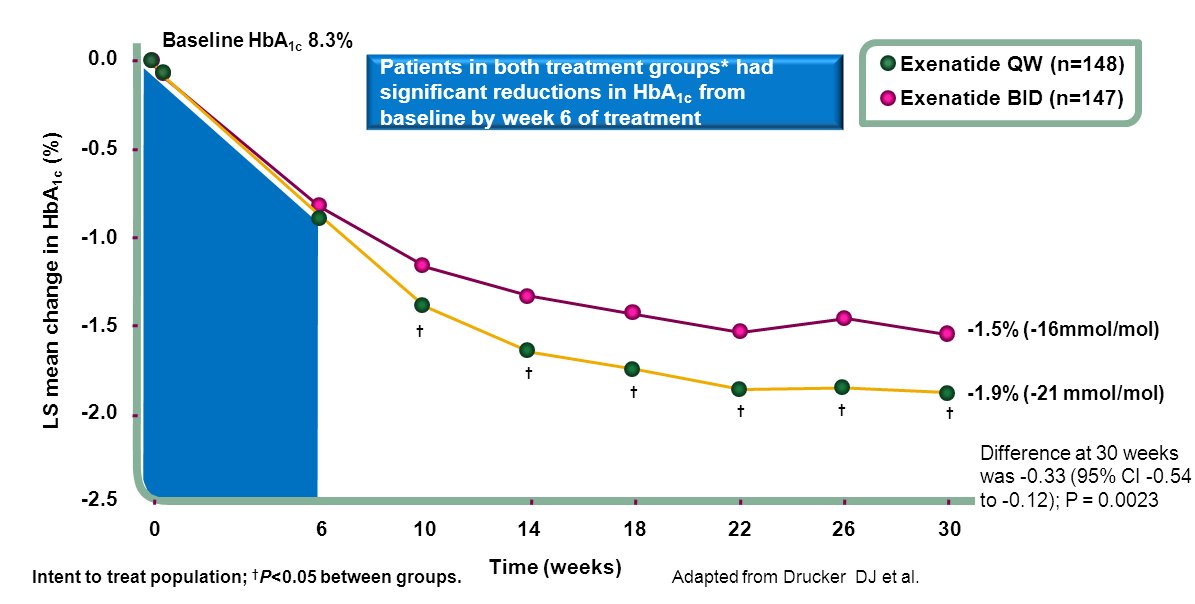
Nationwide exenatide QW audit
The nationwide audit of the once weekly GLP1 receptor agonist, exenatide
QW (Bydureon®) audit launched in April 2014. All users of exenatide QW are
invited to join the audit. The tool provided for on-line data entry
has a facility for easy, sophisticated, analysis of the data entered by
the local centre entering the data. At the same time the data entered
automatically becomes available, in anonymised form, for the national
audit.
Click here for more information and
to join the audit.
Other Nationwide audits of new diabetes therapies
The evolution of the ABCD audits started with a simple home-grown
on-line audit tool for the exenatide audit which evolved into a new tool
for the liraglutide audit which went through 3 iterations to end, in
November 2014, with a secure on-line tool with sophisticated analysis
capability to match the N3 tools mentioned above. The tool for the
nationwide insulin degludec and IDegLira audits were developed from the lirgalutide
audit tool so those who took part in that audit will find it particularly
easy.
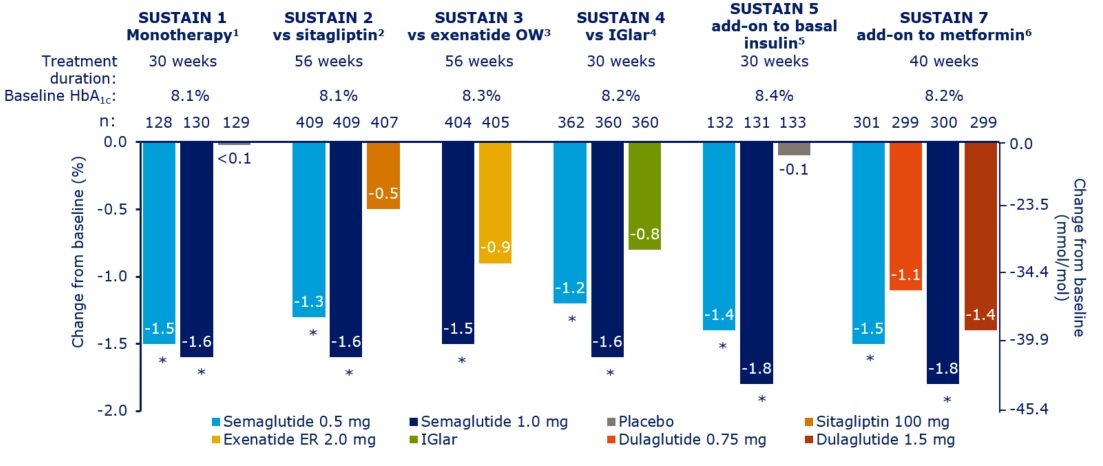
Nationwide semaglutide audit
The nationwide audit of the once weekly GLP1 receptor agonist,
semaglutide (Ozempic®) audit launched in January 2019. All users of
semaglutide are invited to join the audit. The tool provided for on-line
data entry has a facility for easy, sophisticated, analysis of the data
entered by the local centre entering the data. At the same time the data
entered automatically becomes available, in anonymised form, for the
national audit.
Click here for more information and to join the audit.
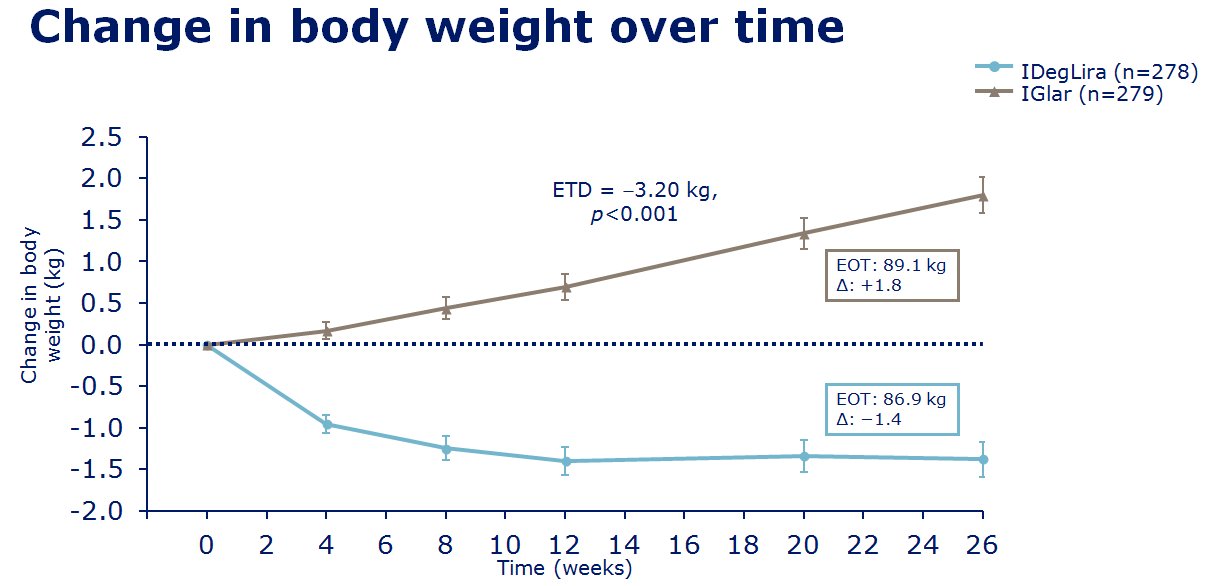
Nationwide IDegLira audit
The nationwide audit of IDegLira (Xultophy®), the fixed combination of
insulin degludec and liraglutide in a single injection, launched in May 2017, at the
Spring ABCD
meeting in Belfast. All users of IDegLira are
invited to join the audit. The tool provided for on-line data entry
has a facility for easy, sophisticated, analysis of the data entered by
the local centre entering the data. At the same time the data entered,
automatically becomes available, in anonymised form, for the national
audit.
Click here for more information and
to join the audit.
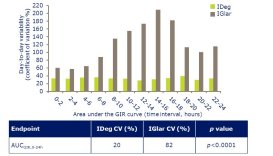
Nationwide degludec audit
The nationwide audit of the new ultra-long acting insulin analogue,
degludec (Tresiba®) launched in November 2014, at the Autumn ABCD
meeting at the Royal College of Physicians. All users of degludec are
invited to join the audit. The tool provided for on-line data entry
has a facility for easy, sophisticated, analysis of the data entered by
the local centre entering the data. At the same time the data entered,
automatically becomes available, in anonymised form, for the national
audit.
Click here for more information and
to join the audit.
|