|
ABCD nationwide semaglutide audit
About the ABCD nationwide
semaglutide
audit
This audit follows on from the success of the
previous ABCD
nationwide audits of GLP1 receptor agonists, SGLT2 inhibitors,
insulin degludec, IDegLira and most recently the FreeStyle Libre. The
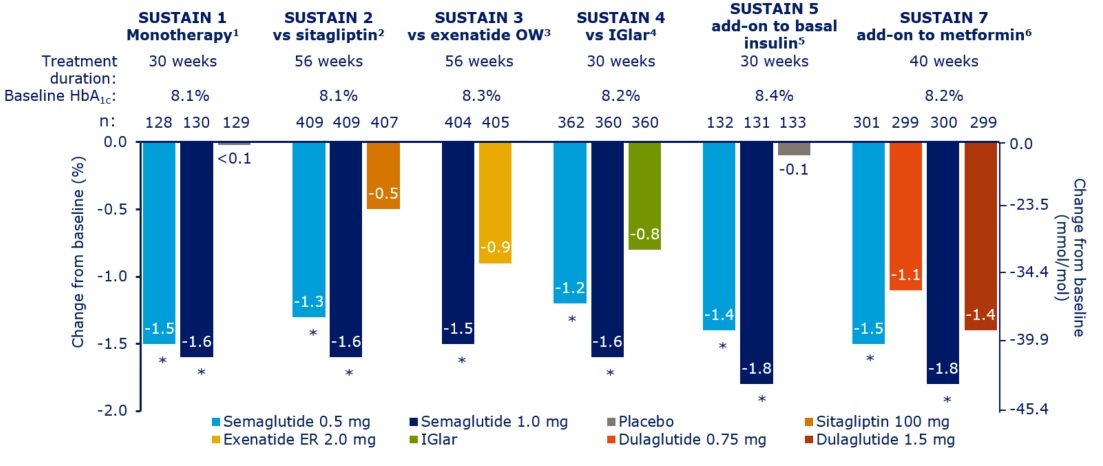
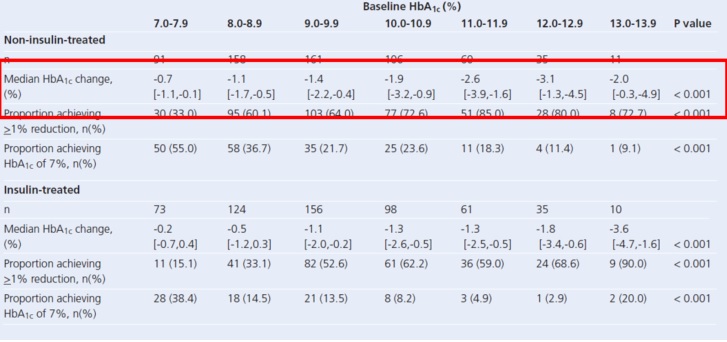
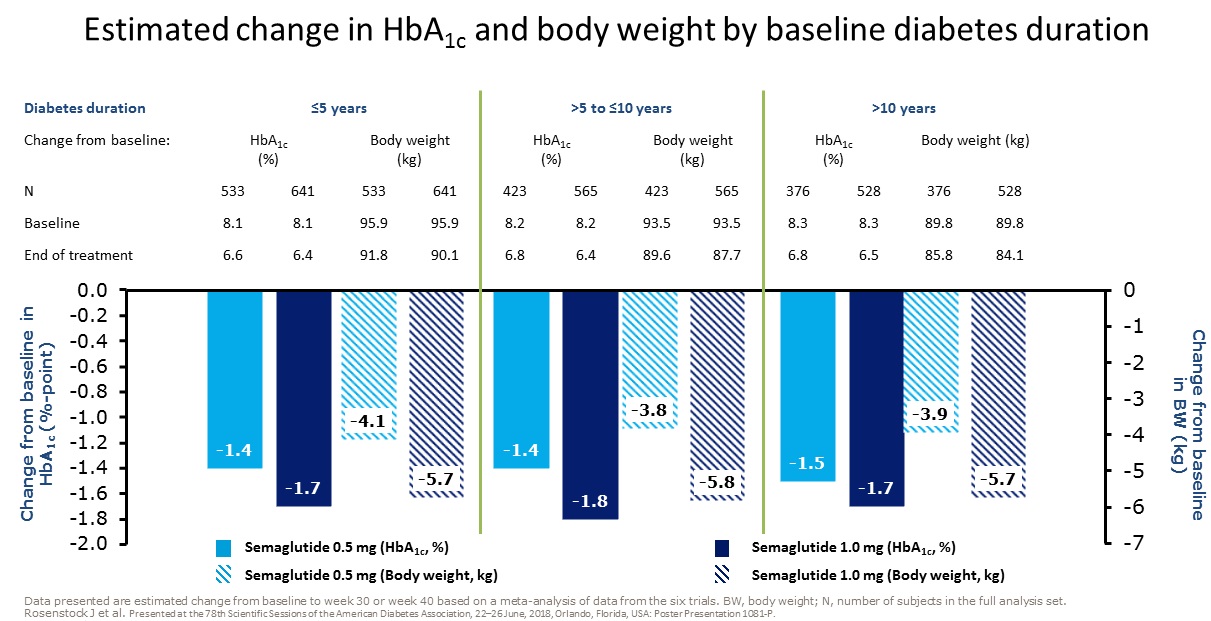
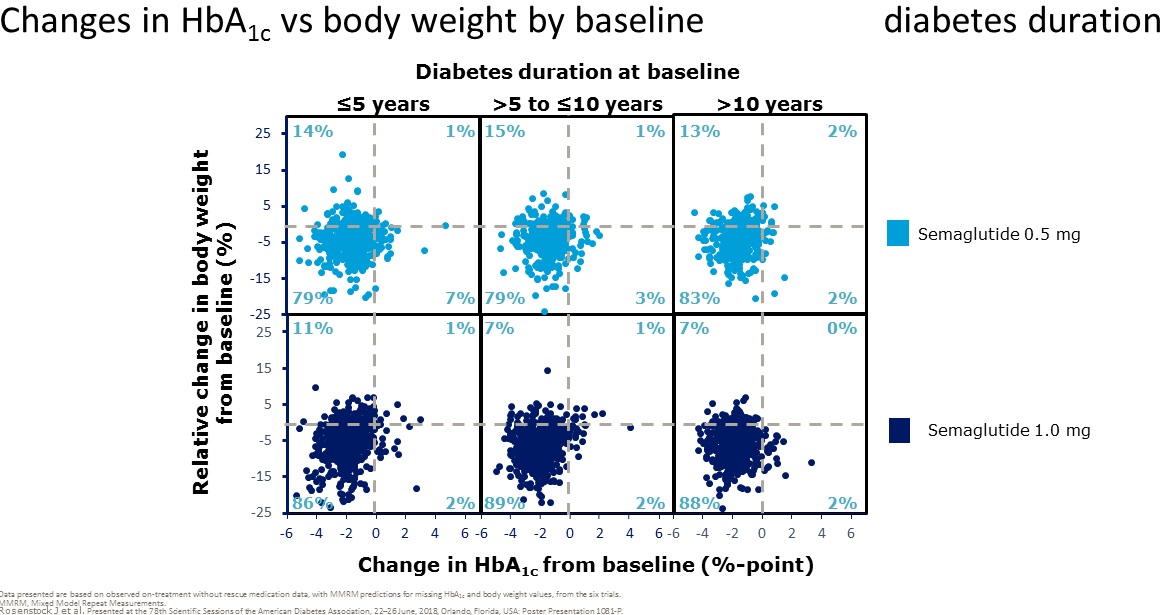
clinical trials with semaglutide suggest that it is
more effective
than the other GLP-1 receptor agonists we have had to date and it is
now of interest for us to find out the extent to which the experience in
clinical trials translates into the real world. There is no information
from the clinical trials regarding the impact of switching from other
GLP-1 receptor agonists to semaglutide - we will learn this from our
audit, along with the other points of interest in the left hand column
of this page - and much else. In the
SUSTAIN 6 trial semaglutide seemed to have a bigger impact on
cardiovascular outcomes than previously studied diabetes medications
which may increase its usage in view of the
consensus report by the American Diabetes Association (ADA) and the
European Association for the Study of Diabetes (EASD) suggesting
that agents with proven cardiovascular benefit should be used more
readily in type 2 diabetes patients with established atherosclerotic
cardiovascular disease. The audit will be hosted on a tool very similar
to that used in the
liraglutide audit, the
degludec audit and the
IDegLira audit so the many contributors taking part in those audits
will find it particularly easy. The
audit launched in January 2019 and has a number of
objectives.
Collect
data on-line or via paper forms
The semaglutide on-line audit tool is so
easy to use that live data entry in clinic is a real option
to be considered. Otherwise to facilitate data collection
during clinics there are two paper forms which exactly match
the data that can be entered into the audit tool. You can
download and print these forms locally.
To download the forms to printout for use, use the
following links:
Download first visit data entry form
Download follow up visit data entry form
Non ABCD members
Non ABCD members are welcome to take part in the audit and will be given
access to the on-line audit tool when they
register for the audit.
Analyse your own data
The tool will allow you to analyse the data of your own patients for
your own local interest; at the same time the data will automatically be
available for national analysis of anonymised data.
A video showing
how to easily analyse your data is available on the ABCD YouTube
channel.
Acknowledgement of contributors
As we have done with previous audits all contributors will be
acknowledged in all papers and presentations from the audit data and
biggest contributors will be offered the possibility of being
co-authors.
Register to take part in the audit and
access to the on-line tool
To
register for the audit and be given access to the on-line tool on the
ABCD website
click here.
Further information
Further enquiries may be made to the ABCD nationwide audits database
administrator of the project,
Melissa Cull. |