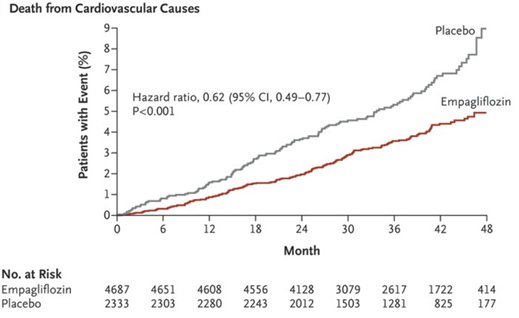
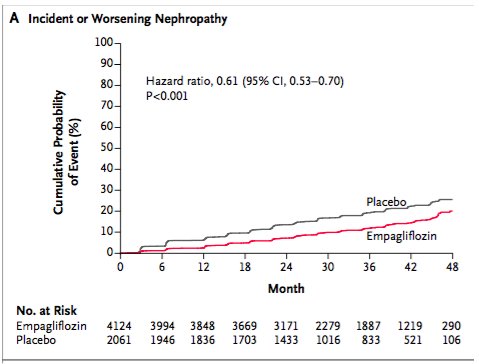
|
|
ABCD nationwide empagliflozin audit on N3
About the ABCD nationwide
empagliflozin
audit
With the launch of the
empaagliflozin audit in March 2017, ABCD is now auditing all three from new class of drugs
for diabetes, the SGLT2 inhibitors. As with the ABCD GLP1-receptor
agonist audits (exenatide,
liraglutide and
exenatide QW) it may be
that patients being treated with the SGLT2 inhibitors in real clinical
practice will be different from those in clinical trials and outcomes
may also be different both in terms of safety and efficacy. As with the exenatide QW, dapagliflozin
and canagliflozin audits, this audit is again being hosted
on N3 - the latest version of NHSnet. Access the on-line tool for the
audit does require the user to be on N3 – ie in an NHS hospital, GP
surgery etc. The audit has a number of
objectives.
Web-based audit tool on N3
The audit tool for the empagliflozin audit is very similar to that being
used for the dapagliflozin and canagliflozin audits. This will make comparison between the
data in the three audits and combining data for analysis of class an easy
task. The tool is easy to use. Being on N3 it has optimum security for
patient identifiable data with regard to your own patients, but anonymises the data when it is utilised in the national audit. There are
some special features with regard to the data export both for your own
local analysis and for the nationwide analysis. The export now allows
you to choose which data to download for analysis as well as providing
all data. It also allows you to choose to download the data aggregated
to 2 monthly, 3 monthly, 4 monthly or 6 monthly time points. The tool
has the facility to detect data from the same patient entered in two
sites (eg hospital and primary care) and to merge the data when exported
- see centres and sites below. As with the canagliflozin audit, to improve patient safety,
Boehringer Ingelheim's pharmacovigilance
team will be alerted automatically of any serious adverse events. This
is in addition to the usual advice that adverse events occurring in the
UK should be reported to the yellow card scheme:
www.mhra.gov.uk/yellowcard.
Structure of the audit –
centres and sites
For this audit the concept of centres and sites is utilised in the same
way as in the exenatide QW, dapagliflozin and canagliflozin audits. Typically a centre
might be an NHS Trust. Sites might be hospitals associated with that
Trust, and/or health centres or GP surgeries in the local vicinity. If
set up in this structure, designated leaders of the local audit would be
given access to download the anonymised data of all the patients
associated with the centre for more powerful local analysis of data
involving higher numbers. Findings so made through such local analysis
could be put forward for further testing on the full national dataset.
Collect data on-line or
via paper forms
The on-line audit tool is so easy to use that live data entry in clinic
is a real option to be considered. Otherwise to facilitate data
collection during clinics there are two paper forms which exactly match
the data that can be entered into the audit tool. You can download and
print these forms locally or
order pre-printed data entry forms.
To download the forms to printout for use, use the following links:
Download first visit data entry form
Download follow up visit data entry
form
Non ABCD members
Non ABCD members are welcome to take part in the audit and will be given
access to the on-line audit tool when they
register for the audit.
Register to take part in
the audit and access to the on-line tool
To register for the audit and be given access to the on-line tool on the
ABCD website on N3
click here.
Further information
Further enquiries may be made to the ABCD nationwide audits database
administrator of the project,
Melissa Cull |
Register for the
empagliflozin audit: UK
Register
simultaneously for ALL 3
SGLT2 audits: canagliflozin, dapagliflozin and
empagliflozin
Access
the on-line tool
(UK: you need to be on N3)
Empagliflozin audit objectives
Order
preprinted data entry forms
Download
first visit data entry form
Download
follow up visit data entry form
How
to analyse your data - video
Papers, abstracts, presentations, posters from the audit
Further
information- contact us
Main ABCD homepage
|